Carbon Dating
❤️ Click here: How does carbon dating help scientists learn about early cultures
An illustration may help: Imagine you found a candle burning in a room, and you wanted to determine how long it was burning before you found it. For example, a jar starting with all 14C atoms at time zero will contain half 14C atoms and half 14N atoms at the end of 5,730 years one half-life.

For biological objects older than 50,000 years, scientists use radioactive dating to determine the age of rocks surrounding where the material was found. However, Carbon dating is at best a good theory, and thatis all it is, a theory.
Carbon-14 dating - At the time rocks form, however, their magnetic materials acquire the particular orientation of the planet's magnetism at the time, giving geologists a window into the Earth's magnetic past.

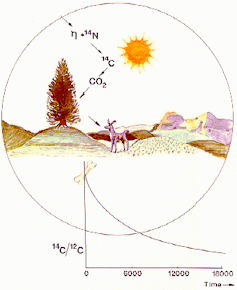
Radioactive dating uses the decay rates of radioactive substances to measure absolute ages of rocks, minerals and carbon-based substances, according to How Stuff Works. Scientists know how quickly radioactive isotopes decay into other elements over thousands, millions and even billions of years. Scientists calculate ages by measuring how much of the isotope remains in the substance. The key to an age of a substance is the decay-product ratio. The ratio of the original isotope and its decay product determines how many half-lives have occurred since the sample formed. A half-life measures the time it takes for one half of a radio isotope's atoms to break down into another element. For instance, if an object has 50 percent of its decay product, it has been through one half-life. A popular way to determine the ages of biological substances no more than 50,000 years old is to measure the decay of carbon-14 into nitrogen-14. This process begins as soon as a living thing dies and is unable to produce more carbon-14. Plants produce carbon-14 through photosynthesis, while animals and people ingest carbon-14 by eating plants. Carbon-14 has a half-life of 5,730 years. Scientists determine the ages of once-living things by measuring the amount of carbon-14 in the material. For biological objects older than 50,000 years, scientists use radioactive dating to determine the age of rocks surrounding where the material was found. By dating rocks, scientists can approximate ages of very old fossils, bones and teeth. Radiocarbon dating was invented in the 1940s by Willard F. Radioactive dating is used in research fields, such as anthropology, palaeontology, geology and archeology.
Modern Humans’ Earliest Artwork and Music: New European Discoveries
However, there is strong evidence which suggests that radioactive decay may have been greatly accelerated in the unobservable past. By contrast, radiocarbon dating provided the first objective dating method—the ability to attach approximate numerical dates to organic remains. After the organism dies it stops taking in new carbon. What methods do they use and how do these methods work. To measure the amount of radiocarbon left in a artifact, scientists burn a small piece to convert it into carbon dioxide gas. Precise measurements taken over the last 140 years have met a steady decay in the strength of the earth's magnetic field. This means its nucleus is so large that it is unstable. Ice sheets are laid down in layers, and the layer corresponding to each year is a little different. If the production rate of 14C in the resistance was less in the past, dates given using the carbon-14 method would incorrectly assume that more 14C had decayed out of a specimen than what has actually occurred.



